Introduction
Central retinal artery occlusion (CRAO) is an ophthalmic emergency that is analogous to a cerebral stroke. It is caused by the sudden blockage of the central retinal artery (CRA) and usually presents as sudden, painless monocular vision loss.
Importantly, the prognosis for overall spontaneous visual recovery in CRAO is low, as it has been found that only 17.7% of patients obtain functional visual recovery without any treatment.
Furthermore, CRAO indicates end-organ ischemia, often due to underlying atherosclerosis. These underlying atherosclerotic risk factors also increase the risk of future cerebral stroke and ischemic heart disease in the individual.
There is currently no guideline-endorsed evidence for treatment of CRAO. As CRAO is the ocular analog of a cerebral stroke, the clinical approach and management are similar to stroke management.
Management
The principles of management of CRAO can be divided into:
-
Acute: Restore blood flow
-
Subacute: Prevention of secondary complications
-
Secondary prevention: Systemic control and prevention of future vascular ischemic events.
Acute management of Central retinal artery occlusion (CRAO)
CRAO can cause abrupt and often irreversible visual loss unless retinal circulation is re-established acutely. There is no consensus in the management of acute CRAO, and various treatment options have been advocated.
Current literature suggests that acute management can be divided into noninvasive or invasive therapy.
The retinal tolerance time has been studied in various experimental analyses. A study of atherosclerotic and hypertensive rhesus monkeys has suggested that no detectable damage occurs with CRAO within 97 min, but occlusion lasting for 240 min or longer can cause irreversible damage.
This suggests that there may be a retinal tolerance time within 240 min and therefore any acute treatment should be provided within 4 h.
However, the exact time when the damage of CRAO can be reversed is unknown and has been postulated to be in between 4.5 and 12 h.
Previously, there were two randomized controlled trials investigating the use of thrombolysis in a 24-hour window intravenously and intra-arterially but did not show any clinically significant outcome.
A recent meta-analysis showed that the administration of intravenous (IV) alteplase within 4.5 h of symptom onset is associated with a higher likelihood of a favorable visual outcome for acute CRAO.
Noninvasive therapies include:
-
Vasodilation of the CRA
-
Reducing the intraocular pressure
-
Reducing retinal edema.
Vasodilation of the central retinal artery
Dilation of the retinal artery can increase the blood flow and hence may enable the removal of the CRA blockage. A potential risk of vasodilation includes systemic vasodilation which causes a decrease in systemic blood pressure.
The various vasodilators that have been described as useful in the management of CRAO include sublingual isosorbide dinitrate, inhaling carbogen (95% oxygen and 5% carbon dioxide), hyperbaric oxygen treatment (HBOT), and pentoxifylline.
Sublingual isosorbide dinitrate causes relaxation of the vascular smooth muscle and can affect the ocular blood flow.
However, isosorbide dinitrate has only been used in CRAO as a combination treatment with ocular massage or other means to reduce intraocular pressure.
Inhaling carbogen is used for CRAO treatment by maintaining or improving the blood flow while maintaining the oxygenation of the retina by preventing oxygen-induced vasoconstriction.
It has been proposed that carbogen inhalation be used for 10 min every waking hour and 4 hours during the night for a total of 48–72 hours.
Later studies have suggested that the visual recovery with carbogen in patients with CRAO is not significant.
HBOT includes 100% oxygen inhalation to increase the amount of oxygen dissolved in body tissue. In a recent study, HBOT was given at 90-minute sessions three times in the first 24 hours, followed by once daily until there was no further improvement noted in the vision after 2 consecutive treatments.
It was found that HBOT is very effective in CRAO, provided the macular is not ischemic and has not developed cherry-red spots, with the greatest improvement seen if patients are treated within 12 hours of the onset of symptoms.
A recent meta-analysis suggested that HBOT does not improve final visual outcomes and the risks associated include barotrauma, tympanic membrane rupture, and generalized seizures due to oxygen toxicity to the central nervous system.
Vasodilators such as pentoxifylline, which is a xanthine derivative, can help reduce blood viscosity and therefore increase vascular flow and tissue perfusion.
In a small, randomized study of ten patients with CRAO, an improvement in the peak systolic and diastolic flow was noted in the CRA. However, the study was small and the visual recovery was not published by the authors.
Reducing the intraocular pressure
As mean ocular perfusion pressure is the difference between mean arterial pressure and intraocular pressure, reduction in the intraocular pressure will increase the ocular perfusion pressure or help to dislodge the embolus.
The various procedures used to reduce intraocular pressure include ocular massage, the use of IV acetazolamide, the use of IV mannitol, or the use of topical antiglaucoma drops.
Ocular massage can be performed with direct digital pressure or using a contact lens. The compression of the globe is performed for 10–15 s, followed by a release to obtain retinal artery pulsation of flow.
Alternatively, continued digital massage can be applied over closed eyelids for 15–20 min. Combined ocular massage with acetazolamide can reduce intraocular pressure up to 5 mmHg within a short period of time.
Ocular massage causes retinal artery dilatation with fluctuation in intraocular pressure, and this activity may in turn mechanically dislodge an embolus into peripheral retinal circulation.
IV acetazolamide (500 mg), 20% IV mannitol (1.5–2 g/kg body weight), and topical antiglaucoma drops aim to lower the intraocular pressure and increase the pressure gradient across the optic nerve.
Most reports supporting the use of these medications are case reports or series used in conjunction with other treatments rather than as a single agent in the acute management of CRAO.
Reducing retinal edema
The use of intravenous methylprednisolone to reduce retinal edema is a controversial issue. It was reported in a single case series.
However, the patients were much younger, and it is possible that the CRAO was secondary to vasculitis rather than atherosclerotic-induced CRAO. IV methylprednisolone is, however, the treatment of choice in patients with suspected arteritic CRAO due to giant cell arteritis.
These acute therapies, singly or in combination, do not alter the clinical outcome.
Invasive therapies include:
-
Ocular:
-
Anterior chamber paracentesis:
Anterior chamber paracentesis will enable a reduction in the intraocular pressure. The mean perfusion pressure across the optic nerve head is the mean arterial pressure minus the intraocular pressure.
Therefore the paracentesis hopes to increase the perfusion across the optic nerve head. This is performed under topical anesthesia using a 27-gauge needle to drain 50 μL of aqueous fluid at a time under aseptic precautions to achieve sufficient intraocular pressure lowering and maintaining the anterior chamber depth. The potential risks of paracentesis include inadvertent ocular trauma, intraocular hypotony, corneal decompensation due to iridocorneal touch, and infection. A retrospective analysis from Germany suggested little improvement in the vision with anterior chamber paracentesis.
-
Transluminal Nd: YAG laser:
Intraluminal embolus, if visible, is lysed using Nd: YAG laser, starting from low energy (0.5–1 mJ) and increasing in small increments until fragmentation, movement of the embolus into the vitreous, bleeding, or a gas bubble is seen.
The results on the visual prognosis vary from 20% to 50%, achieving a final visual acuity of 20/60.
However, the laser is associated with significant complications including vitreous hemorrhage requiring surgery. Most of these reports are from case series, and a randomized control trial would be beneficial to conclude the significance of Nd: YAG laser in the treatment of CRAO.
-
Pars plana vitrectomy:
Pars plana vitrectomy and removal of embolus have been reported in some cases and case series; however, the studies are limited, and further randomized control studies are required to ensure the safety of this procedure.
-
IV and intra-arterial (IA) tissue plasminogen activator (tPA):
TPA is a naturally occurring thrombolytic agent that is approved for use in patients with acute ischemic syndrome, pulmonary embolism, and acute myocardial infarction.
tPA is highly effective if used within the therapeutic window. Thrombolytic agents can be used to lyse the fibrin platelet thrombus in nonarteritic ischemic CRAO, which is similar to the treatment of acute ischemic stroke. In the management of CRAO, tPA can be administered intravenously or intra-arterially.
-
Intravenous tissue plasminogen activator:
IV tPA, at 0.9 mg/kg body weight (maximum 90 mg with 10% IV bolus and the remainder over 1 h), is given in patients with CRAO within 1.5–4.5 h of the onset of symptoms, as per the recommendation for management of acute stroke.
Of these studies, there has only been one randomized control study that analyzed the effect of tPA in CRAO. In this study, 25% of patients achieved a visual acuity of 3 or more lines within the first week of treatment; however, this improvement was not sustained.
The authors have suggested that this unsustained improvement in the vision could be due to the re-occlusion of the CRA as heparin was not used in the study. In three prospective interventional case series, the use of IV tPA resulted in an improvement in the visual acuity of 32%–55%. The various complications reported include intracranial hemorrhage, ocular hemorrhage, angioedema, abdominal aortic aneurysm, and hematuria.
However, IV tPA, in acute CRAO, is a safer option than IA tPA due to the relatively reduced risk of symptomatic intracranial hemorrhage or ocular hemorrhage.
Three randomized control studies are currently being conducted in Europe to assess the treatment with IV thrombolysis compared with placebo in patients with CRAO presenting within 4.5 h of onset of symptoms:
(a) THEIA (A Phase III Randomized, Blind, Double Dummy, Multicenter Study Assessing the Efficacy and Safety of IV Thrombolysis in Patients with Acute CRAO), (b) REVISION (Early Perfusion Therapy with IV Alteplase for Recovery of Vision in Acute CRAO), and (c) Ten-CRAOS (Tenecteplase in CRAO Study). These trials will provide further information on the benefits and limitations of the use of IV tPA in the management of CRAO.
-
Intra-arterial tissue plasminogen activator:
IA tPA has the advantage of using a reduced dose of the thrombolytic agent while also potentially increasing the time window for administration after developing symptoms. The dosage can be between 10 and 80 mg, with lesser dosage being given as aliquots rather than continuous infusion. Many trials have suggested an improvement in visual acuity in up to 60%–70% of subjects treated with IA tPA.
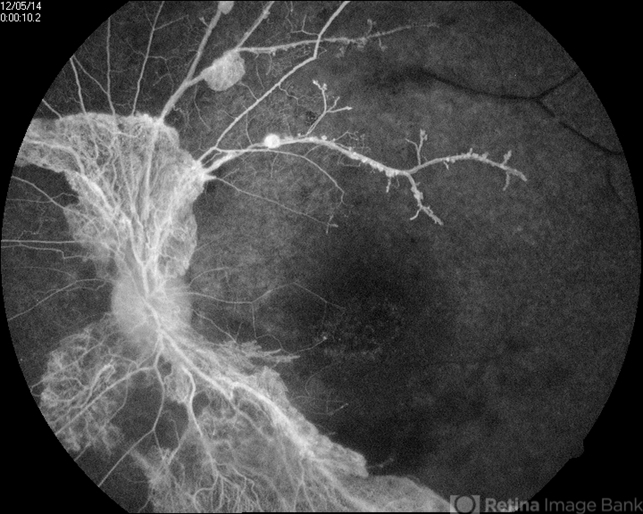
-
-
However, a limitation is that IA tPA must be performed by catheterization of the OA which can only be administered in centers with experienced neuro-interventionalists.
A prospective multicentered randomized controlled trial showed no improvement in visual acuity in patients treated with IA thrombolysis compared to standard treatment. However, concern has been raised with regard to the time of treatment since the time of onset of CRAO and selection bias.
There has been one case series that suggests a vitrectomy followed by a tPA injection to the retinal artery. Both cases were treated within 12 hours of the onset of CRAO and showed an improvement in visual acuity.
The limitation of this study is that it requires intraocular surgery which carries significant ocular complications. Given that this is an individual report, more studies need to be conducted.
Subacute management of Central retinal artery occlusion (CRAO) – preventing secondary ocular complication
CRAO can cause ocular neovascularization due to chronic retinal ischemia resulting from reperfusion failure. Prompt treatment including pan-retinal photocoagulation and neovascular glaucoma should be considered if the patient develops neovascularization.
A direct temporal relationship between CRAO and ocular neovascularization has been reported, with a prevalence of 2.5%–31.6%.
However, Hayreh et al. showed no correlation between CRAO and neovascularization in their study. The patients who did develop neovascularization were found to have no other causes of neovascularization, such as diabetes.
The mean time for neovascularization to be observed post-CRAO was 8.5 weeks (range: of 2–16 weeks). Therefore, neovascularization does occur in CRAO, similar to retinal vein occlusion.
Regular ophthalmology review in the subacute stage is important up to 4 months after CRAO to prevent local complications
Secondary prevention: Systemic control and prevention of future vascular ischemic events Patients with CRAO have an increased risk of future ischemic events.
Therefore, a multidisciplinary team involving an ophthalmologist, neurologist, and a primary care physician or internist is important for long-term secondary prevention in patients with CRAO.
Sixty-four (64%) patients after CRAO had at least one undiagnosed vascular risk factor, with hyperlipidemia being the most common (36%).
Systemic atherosclerotic risk factors should be addressed through medical treatment, diet counseling, and lifestyle modification in order to decrease the risk of future ischemic disease.
This includes treatment of hypertension, hyperlipidemia, obesity, and obstructive sleep apnea. A plant-based diet, regular physical activity, and smoking cessation are also important modifications for secondary prevention after CRAO.
The recommendation for antiplatelet therapy in CRAO parallels the guides from the American Heart Association for transient ischemic attacks or minor strokes.
In the patients without contra-indication, an initial course of 21 days of dual antiplatelet therapy may be reasonably followed by long-term treatment with a single antiplatelet agent, typically aspirin 81 mg daily or clopidogrel 75 mg daily, as recommended by current guidelines.
References
RETINAL IMAGING BY YOUR SMARTPHONE