CASE REPORT
A 65-year-old male presented to an ophthalmology clinic with progressive vision loss in his left eye, accompanied by eye pain, redness, and intermittent blurred vision.

He denied recent trauma or systemic illnesses but reported a 15-year history of well-controlled diabetes mellitus.
Ocular examination revealed visual acuity of 20/200 in the left eye, elevated intraocular pressure (45 mmHg), mild conjunctival injection, and ciliary flush.
Slit-lamp biomicroscopy identified neovascularization of the iris, microhyphema, and anterior chamber cells. Gonioscopy revealed neovascularization of the angle, and fundus examination, limited by corneal edema, showed disc pallor and attenuated vessels.
Ancillary tests, including fundus fluorescein angiography (FFA) and optical coherence tomography (OCT), confirmed leakage from abnormal vessels and vitreoretinal traction, respectively. Neovascular glaucoma (NVG) diagnosis was confirmed.
Neovascular Glaucoma DISEASE entity
Neovascular glaucoma (NVG) is a severe form of secondary glaucoma characterized by the proliferation of fibrovascular tissue in the anterior chamber angle.
Since Coats first noted new vessel formation on the iris (rubeosis iridis) in eyes with central retinal vein occlusion in 1906, this condition has been noted previously by names including hemorrhagic glaucoma, congestive glaucoma, thrombotic glaucoma, and rubeotic glaucoma.
Weiss and colleagues introduced the term neovascular glaucoma in 1963. The common denominator predisposing to this condition is usually retinal ischemia, although some cases are associated with other ocular or extraocular entities.
Neovascular glaucoma (NVG) runs an aggressive clinical course and the condition is usually refractory to medical therapy alone. Surgical approaches to managing this complicated form of glaucoma have evolved over the past few decades while often still resulting in a guarded visual prognosis.

In light of the association of vascular endothelial growth factor (VEGF) with retinal ischemia, the advent of anti-VEGF drugs provides a welcome addition to the treatment strategy for this potentially devastating condition.
Rubeosis iridis and Neovascular glaucoma (NVG) have been associated with a wide range of conditions. Of these conditions, retinal ischemia accounts for the majority of the causes, with central retinal vein occlusion (CRVO) and diabetes retinopathy underlying nearly two-thirds of all Neovascular glaucoma (NVG) cases.
Retinal ischemia triggers a cascade of events beginning with an inadequate oxygen supply to the retinal cells leading to the release of various angiogenic factors including VEGF and interleukin-6.
Normally VEGF levels are in equilibrium with pigment epithelium-derived growth factor (PEDF), an antiangiogenic factor. When the equilibrium between VEGF and PEDF is shifted in favor of VEGF, this promotes activation, proliferation, and migration of endothelial cells, leading to neovascularization of the anterior segment.
Increased levels of Interleukin-6 have also been noted in the aqueous humor of patients with neovascular glaucoma due to central retinal vein occlusion.
MANAGEMENT of Neovascular Glaucoma
Adequate treatment of retinal ischemia with pan-retinal photocoagulation (PRP) is essential in reducing the stimulus for neovascularization of the anterior segment, which may prevent the need for additional surgery.
Treatment of underlying systemic disease may improve neovascularization of the iris, as is the case with endarterectomy for carotid occlusive disease and ocular ischemia.
The advent of anti-VEGF agents has led to their use in the form of intravitreal injections before PRP and/or surgical IOP control.

The duration of suppression of iris and angle neovascularization lasts approximately 3-6 wks with anti-VEGF injections, thereby creating a window of opportunity to allow adequate PRP and/or glaucoma surgery to be carried out.
Medical therapy:
The selection of specific agents to lower IOP depends upon the stage of NVG. Because elevated IOP is in part due to compromised trabecular meshwork function, aqueous suppressants (beta blockers, alpha-agonists, carbonic anhydrase inhibitors) should theoretically have the greatest efficacy.
Prostaglandins may also be effective, and while a theoretical risk for exacerbating ocular inflammation exists, no evidence to date supports this in Neovascular glaucoma (NVG).
Because aqueous suppressants are often insufficient to achieve IOP control, prostaglandin analogs should be used as needed. Miotic agents or any other medications acting on aqueous outflow through the conventional pathway are least likely to be effective if the angle is already closed.
Osmotic agents may be used to clear the cornea to improve visualization for treatment or diagnosis. Adjunctive treatment with a short course of topical steroids may control inflammation to improve the outcome of subsequent surgery, and cycloplegics may aid in patient comfort and improve visibility for PRP.
Surgery:
In eyes with vision better than 20/400, most glaucoma specialists prefer glaucoma drainage implant placement or filtering surgery versus cyclophotocoagulation.
Regardless of the surgical procedure chosen, preoperative pan-retinal photocoagulation should be performed whenever possible.

The presence of florid neovascularization poses a significant risk for intraoperative and postoperative hemorrhage, either extra- or intraocular.
While trabeculectomy offers the advantage of achieving lower postoperative IOP compared to aqueous shunts, failure to adequately resolve active neovascularization will lead to bleb failure through conjunctival scarring at the filtration site.
Aqueous shunts bear the advantage of avoiding the necessity of surgical iridectomy, thereby decreasing the risk of intraoperative hemorrhage.
In addition, aqueous shunts are more tolerant of intraoperative and postoperative bleeding and post-operative fibrin reactions.
Therefore, in the setting of active neovascularization, inflammation, and/or hyphema, an aqueous shunt is preferred. In a quiet eye with a view for adequate PRP, trabeculectomy may be considered.
More recently, adjunct treatment with anti-VEGF agents has also shown promise for decreasing intraoperative hemorrhage while improving IOP control and surgical success.
Cyclophotocoagulation is reserved for eyes with poor visual prognosis (20/400 or worse) or patients who are poor surgical candidates.
Transcleral diode and YAG techniques are less likely to result in hypotony and phthisis compared to cryotherapy.
HOW TO TAKE SLIT-LAMP EXAM IMAGES WITH A SMARTPHONE?
Smartphone slit-lamp photography is the new advancement in the field of science and technology in which photographs of the desired slit-lamp finding can be taken with smartphones by using the slit-lamp adapters.
Slit-lamp Smartphone photography
REFERENCES
- Wand M., Neovascular glaucoma. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas – Clinical Science. 2nd. Ed. St. Louis: Mosby; 1996: 1073-1129. Ch 51.
- Coats G., Further cases of thrombosis of the central vein. Roy Lond Ophthal Hosp Rep 1906; 16:516.
- Weiss DI, Shaffer RN, Nehrenberg TR Neovascular glaucoma complicating carotid-cavernous fistula. Arch Ophthalmol. 1963; 69:304-307.
- Sivak-Callcott JA, O’Day DM, Gass JD, et al. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001; 108:1767-1776.
- Hayreh SS., Neovascular glaucoma. Prog Retin Eye Res. 2007; 26(5): 470-485.
- Simha A, Aziz K, Braganza A, Abraham L, Samuel P, Lindsley KB. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev. 2020 Feb 6;2(2):CD007920.
- Tamura T. Electron microscopic study of the small blood vessels in rubeosis iridis diabetica. Jpn J Ophthalmol 1969;13:65.
Slit-lamp Smartphone photography
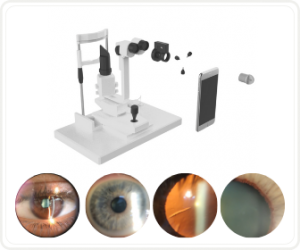
RETINAL IMAGING BY YOUR SMARTPHONE