CASE REPORT
A 58-year-old male with a history of hypertension and hyperlipidemia, as well as a previous right-eye cataract surgery three years ago, presented with a six-month history of gradually decreasing vision in his left eye, accompanied by the perception of “floaters,” flashes of light, and central vision blurriness.

Initial clinical assessment revealed reduced visual acuity in the left eye (20/200), and mild inflammatory signs on slit-lamp examination.
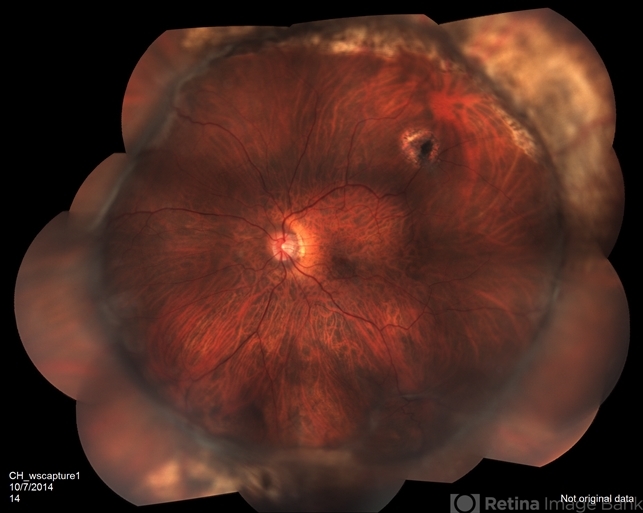
Fundus examination confirmed the presence of a partial retinal detachment in the inferior quadrants, characterized by retinal folds and multiple pigment epithelial changes.
Optical coherence tomography (OCT) further substantiated the diagnosis of Proliferative Vitreoretinopathy (PVR) based on the clinical presentation and imaging findings.
Proliferative Vitreoretinopathy DISEASE entity
Proliferative vitreoretinopathy (PVR), a major complication of rhegmatogenous retinal detachment (RRD), is an abnormal process whereby proliferative, contractile cellular membranes form in the vitreous and on both sides of the retina, resulting in tractional retinal detachment with fixed retinal folds.
However, it is increasingly being recognized that PVR may be intraretinal, which causes retinal shortening. Research suggests that membranes form in response to cytokines and inflammatory mediators that arise following anatomic disruption and tissue damage caused by rhegmatogenous retinal detachment (RRD) and resultant inflammation.
Treatment is principally surgical and often requires multiple procedures that, in fact, yield a high rate of retinal reattachment; nevertheless, many anatomically successful eyes do not recover good visual function likely due to the long-standing macular detachment.
Proliferative Vitreoretinopathy (PVR) formerly named “massive vitreous retraction” and “massive periretinal proliferation” — describes the aberrant process whereby epi/subretinal membranes form following rhegmatogenous retinal detachment (RRD), ultimately leading to retinal traction and recurrent retinal detachment.
Intraretinal PVR is caused by glial tissue that is activated to proliferate within the retina and can cause retinal shortening. PVR arises in an estimated 5-10% of RRD cases and therefore represents a major complication of retinal detachment.
Proliferative Vitreoretinopathy Differential diagnosis
When assessing a patient for PVR, one should also consider a variety of other proliferative and contractional retinal diseases, in addition to other fibrosing conditions, including:
- Proliferative vascular retinopathy (proliferative diabetic retinopathy, other proliferative ischemic retinopathies, and retinopathy of prematurity)
- Retinal detachment following open globe ocular trauma
- Retinal detachment following intraocular foreign body
Rarely, a RRD without PVR can have extensive folds that falsely appear to be fixed.
Seen mostly in highly myopic eyes with very thin retinas, the retina in this instance remains normally mobile on eye movement, no membranes are found at surgery and the retina completely flattens following surgery.
MANAGEMENT of Proliferative Vitreoretinopathy
Proliferative Vitreoretinopathy (PVR) is primarily managed through pars plana vitrectomy and membrane peeling, although these procedures are rarely necessary in the case of grade A or B PVR.

While some cases of grade C PVR can be managed with a scleral buckle without vitrectomy, most will require vitrectomy and membrane peeling. If an encircling scleral buckle is present, it is usually left in place.
If no scleral buckle is present, an encircling scleral buckle is usually placed in order to help reduce residual peripheral traction sometimes present even after extensive membrane peeling.
This procedural combination of vitrectomy, membrane peeling, and scleral buckle is done to alleviate the retinal traction in PVR and facilitate retinal flattening.
Vitrectomy can be performed with any size vitrectomy instrumentation, although most surgeons currently use 25- or 23-gauge micro-vitrectomy systems. Membrane peeling is usually done with a pick and end-grabbing forceps.
Some membranes can be grasped with forceps and removed, while some will require elevation with a pick. Note that mature membranes are often easily removed in a continuous sheet, as opposed to immature membranes that may tear more readily and split.
A bimanual approach with the pick in one hand and the forceps in the other hand is the preferred method of membrane peeling, although some surgeons prefer to use two forceps for bimanual membrane peeling.
Illumination during membrane peeling can be provided by an illuminated pick (a fiberoptic light with an attached pick) or by a “chandelier” (a stationary fiberoptic light placed through another cannula at the pars plana).
Peeling is usually started in the posterior retina and progresses anteriorly. In eyes with anterior PVR, membrane peeling can be difficult to adequately relieve traction. After posterior membranes are removed, perfluorocarbon liquid (PFCL), which is heavier than water or saline, can be used to weigh down and stabilize the posterior retina to ease dissection of the mid-peripheral membranes and the retina pulled anteriorly and adherent to membranes at the vitreous base or ciliary body.
As a last resort, it is sometimes necessary to perform a relaxing retinectomy to relieve traction. The relaxing retinectomy is a circumferentially cut in the peripheral retina in the area of traction with excision of the devitalized retina anterior to the cut retina.
Most relaxing retinectomies are done inferiorly where anterior PVR is most commonly found but occasionally will extend 360 degrees. The retinectomy should be as anterior as possible to spare as much posterior, functional retina as possible and should almost always be in a circumferential direction.
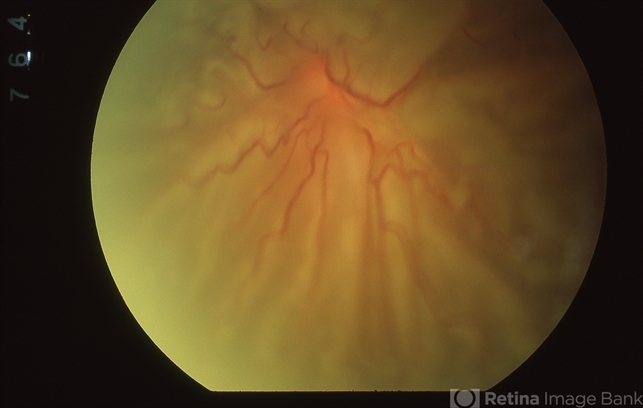
The retinectomy is usually done with the vitrectomy instrument after marking the area and ensuring hemostasis with endo-diathermy. The retinectomy should extend beyond the abnormal area on both sides, otherwise retina may not settle and there will be a risk of subretinal air or perfluorocarbon liquid.
After all, traction is relieved by membrane peeling and, if necessary, a peripheral retinectomy, the retina is usually reattached with PFCL. After reattachment, PFCL can be exchanged for gas or sometimes directly for silicone oil.
Laser treatment delivered through a fiberoptic probe (endo-laser photocoagulation) is used to treat all retinal breaks and usually to place a laser barrier 360 degrees around the peripheral retina (over the scleral buckle if present) and can be performed through PFCL or a long-acting gas.
The benefit of FAX prior to laser is to flatten the anterior detached retina prior to laser. The view can be challenging sometimes through air and then the decision to laser under PFCL is made.
However, air can provide a wider angle improve the visualization of the anterior retina, and also be helpful. The PFCL or air is replaced by long-acting gas (usually perfluoropropane [C3F8] gas) or silicone oil.
For primary PVR in which the eye has not had a prior vitrectomy, C3F8 gas or silicone oil are equally effective. In eyes with prior vitrectomy for PVR and those with a large peripheral retinectomy or with a giant retinal tear more than 90 degrees in circumference, silicone oil may be more effective.
Would you have interest in taking retinal images with your smartphone?
Fundus photography is superior to fundus analysis as it enables intraocular pathologies to be photo-captured and encrypted information to be shared with colleagues and patients.
Recent technologies allow smartphone-based attachments and integrated lens adaptors to transform the smartphone into a portable fundus camera and Retinal imaging by smartphone.
REFERENCES
- Pastor, J. C. Proliferative vitreoretinopathy: An overview. Survey of Ophthalmology 43, 3-18, doi:https://doi.org/10.1016/S0039-6257(98)00023-X.
- Leaver, P. K. Proliferative vitreoretinopathy. The British Journal of Ophthalmology 79, 871-872 (1995).
- Campochiaro, P. A. Pathogenic mechanisms in proliferative vitreoretinopathy. Archives of Ophthalmology 115, 237-241, doi:10.1001/archopht.1997.01100150239014 (1997).
- Charteris, D. G., Sethi, C. S., Lewis, G. P. & Fisher, S. K. Proliferative vitreoretinopathy—developments in adjunctive treatment and retinal pathology. Eye 16, 369, doi:10.1038/sj.eye.6700194 (2002).
- Morescalchi, F., Duse, S., Gambicorti, E., et al. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators of Inflammation 2013, 269787-269787, doi:10.1155/2013/269787 (2013).
- Yu, D. Y. & Cringle, S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Progress in Retinal Eye Research 20, 175-208 (2001).
RETINAL IMAGING BY YOUR SMARTPHONE
RETINAL IMAGING BY YOUR SMARTPHONE