CASE REPORT
A 73-year-old Chinese woman was first diagnosed in the department of neurology with headache, nausea, vomiting, and elevated body temperature. Cerebrospinal fluid (CSF) assays showed significant increases in leukocytes and Cerebrospinal fluid total protein (CS-TP).
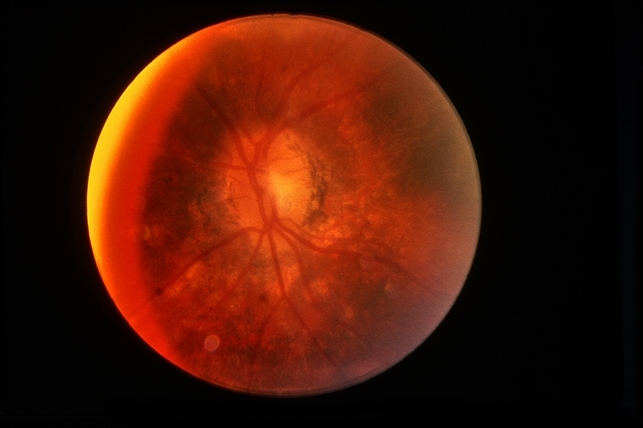
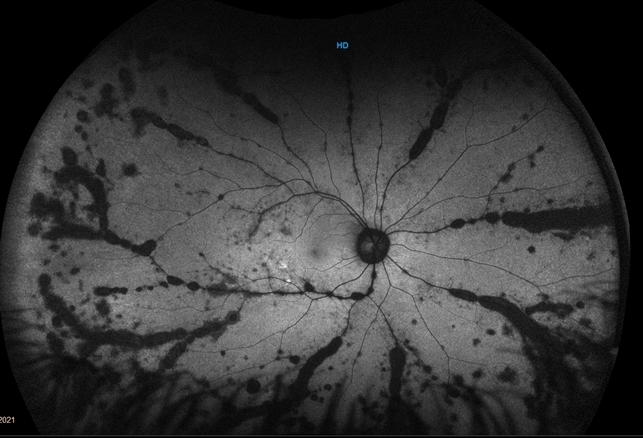
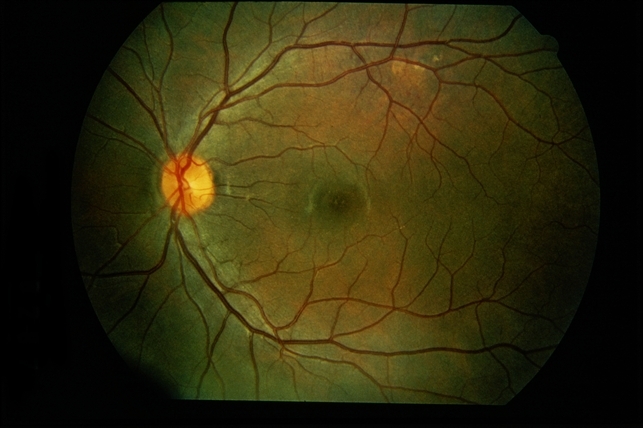
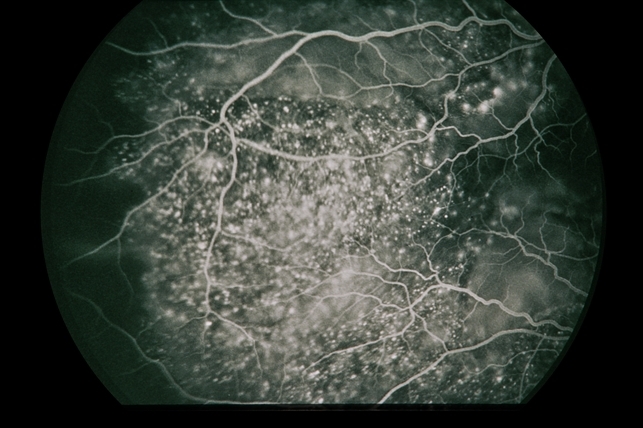
Gradually, symptoms appeared in the eyes including decreased vision, keratic precipitates (KP) (+++), iris local posterior adhesion, vitreous opacity, and optic disc congestion edema. The fundus fluorescence examination showed optic disc hyperfluorescence with both eyes exudative retinal detachment.
Type-B ultrasonography of the obit showed a large number of flocculent opacities in the vitreous and retinal edema. Optical coherence tomography (OCT) showed that significant edema was evident in the macular area and papillary, and there were wavy changes in the retinal pigment epithelium layer.
A review of the clinical, photographic, fundus fluorescence angiography (FFA) data of the patient suggested a clinical diagnosis of Vogt-Koyanagi-Harada Disease (VKH).
Vogt-Koyanagi-Harada Disease (VKH) DISEASE entity
Vogt-Koyanagi-Harada Disease (VKH) is defined as a bilateral granulomatous panuveitis with or without extraocular manifestations affecting young adults.
Originally, Vogt-Koyanagi-Harada Disease (VKH) was classified as two separate entities:
- Vogt-Koyanagi syndrome is characterized by chronic severe anterior uveitis, alopecia, poliosis, cutaneous as well as perilimbal vitiligo (also known as Sugiura’s sign), and dysacusia.
- Harada’s disease is characterized by bilateral exudative uveitis accompanied by pleocytosis of cerebrospinal fluid.
Since there is much overlapping of signs and symptoms between the two entities, in 1932 Babel suggested calling the entity Vogt-Koyanagi-Harada disease.
The incidence of VKH will vary depending on the geographic location and the ethnicity encountered. The disease primarily affects pigmented races. In Japan it accounts for 6.8% to 9.2% of uveitis cases, meanwhile, in the United States, it hovers around 1%-4%.
The majority of the cases found are around the second and fifth decades of life. Women have been reported as being more affected than men, but this will vary depending on the population studied.
MANAGEMENT of Vogt-Koyanagi-Harada Disease (VKH)
In the acute phase, intravenous high-dose corticosteroids (IV methylprednisolone 1g or IV dexamethasone 100mg to run over 1 hr, after excluding systemic infection and contraindications, under physician’s supervision) are usually advised for 3 days followed by high dose oral steroids to be tapered very slowly.
Treatment involves the prompt use of systemic corticosteroids administered orally at a dose of 1-1.5mg/kg per day for a minimum of 6 months. Lai TY et al reported that patients receiving treatment for less than 6 months were more likely to have recurrences (58.8%) compared to those treated for 6 months or more (11.1%).
The initial high dose is maintained for 2-4 weeks followed by gradual tapering of the drug. Many authors differ in what should be considered first-line therapy in Vogt-Koyanagi-Harada Disease (VKH), advocating the use of immunosuppressants as the treatment of choice.
Some of the reasoning behind the use of immunosuppressive therapy is to avoid the many side effects associated with long-term corticosteroid use. Immunosuppressant therapy as first-line treatment has been associated with better visual acuity outcomes when compared to corticosteroid therapy alone.
The immunosuppressants that have proven efficient in clinical trials are Azathioprine (1-2.5mg/kg/day) and Cyclosporine A (3-5mg/kg/day). Mycophenolate mofetil and Rituximab have also been used.
It is important to remember that the administration of immunosuppressants should be closely monitored and evaluated alongside an internist for any complications or side effects from the treatment.
Would you have interest in taking retina images by smartphone?
Fundus photography is superior to fundus analysis as it enables intraocular pathologies to be photo-captured and encrypted information to be shared with colleagues and patients.
Recent technologies allow smartphone-based attachments and integrated lens adaptors to transform the smartphone into a portable fundus camera and Retinal imaging by smartphone.
REFERENCES
- Yanoff Myron and Duker Jay. Yanoff & Duker: Ophthalmology, 3rd Ed. Mosby, 2008
- Agarwal, Anita. Gass’s Atlas of Macular Diseases, 5th Ed. China, Saunders; 2012:998-1002
- Nussenblatt Robert and Whitcup Scott. Uveitis: Fundamentals and Clinical Practice, 4th Ed. Mosby El Sevier; 2010:303-318
- Kanski Jack and Bowling Brad. Clinical Ophthalmology: A Systematic Approach, 7th Ed. El Sevier Saunders; 2011
- Huang Jogn and Gaudio Paul. Ocular Inflammatory Disease and Uveitis Manuel: Diagnosis and Treatment, 1st Ed. Lippincott Williams & Wilkins, 2010
- Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001;131:647–652
- Attia S, Khochtali S, Kahloun R, Zaouali S, Khairallah M. Vogt – Koyanagi – Harada disease. Expert Rev. Ophthalmol. 2012; 7(6):565-585.
RETINAL IMAGING BY YOUR SMARTPHONE
RETINAL IMAGING BY YOUR SMARTPHONE