CASE REPORT
A 35-year-old male came to an ophthalmology clinic with a complaint of vision loss in both eyes. He had noticed that his peripheral vision had been gradually decreasing over the past several years, and he was having difficulty seeing at night.
He also reported that his mother’s brother had experienced similar symptoms and had been diagnosed with retinitis pigmentosa (RP).
Clinical Presentation:
Upon examination, he had bilateral peripheral retinal pigment epithelium (RPE) atrophy with bone spicule formation, consistent with RP. However, his clinical presentation was not entirely consistent with RP. Unlike RP, which typically presents with night blindness, His visual acuity was mildly affected, and he had more central visual field loss. Additionally, the pattern of his RPE atrophy was not typical of RP.
Further Testing:
Given the atypical presentation, He was referred for genetic testing. The results of his genetic testing revealed a pathogenic mutation in the CHM gene, which is associated with choroideremia.
Diagnosis:
Choroideremia is a rare, X-linked recessive retinal dystrophy that causes progressive vision loss. The CHM gene codes for the Rab escort protein 1 (REP1), which is involved in intracellular protein trafficking. Mutations in the CHM gene result in a deficiency of REP1, which leads to degeneration of the retinal pigment epithelium (RPE) and choroid.
Confirmation of Diagnosis:
His diagnosis of choroideremia was confirmed based on his genetic testing results. Further testing, including electroretinography (ERG) and optical coherence tomography (OCT), confirmed the diagnosis and provided additional information on the severity and extent of the retinal degeneration.
Choroideremia DISEASE entity
Choroideremia is an X-linked chorioretinal dystrophy characterized by the diffuse, progressive degeneration of the retinal pigment epithelium (RPE), photoreceptors, and choriocapillaris. It is caused by a mutation in the CHM gene and is the focus of exciting basic and clinical research.
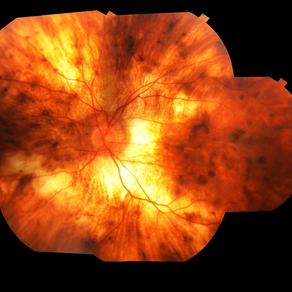
Gene therapy through viral vectors has shown early promise in the possible treatment of this blinding disease. Choroideremia was first described in 1872 by Ludwig Mauthnuer, an Austrian ophthalmologist.
Initially, it was thought to be a developmental anomaly, similar to a choroidal coloboma, due to the near total lack of choroidal vessels. Upon observation of less extreme cases, the progressive nature of the disease became apparent.
An X-linked connection was proposed in 1942, but it wasn’t until 1990 that the specific gene was cloned. The CHM gene was one of the first genes to be identified by positional cloning and was one of the first genes to be established as a cause of inherited retinal degeneration.
Since the early 1990s, over 106 pathogenic variations in the CHM gene have been discovered. The most exciting developments have happened in the last several years, with the development of viral vectors designed to replace the mutated CHM gene.
Diagnosis of Choroideremia
Fundus Examination:
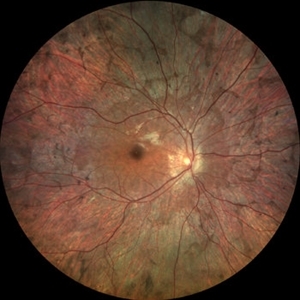
On fundus examination, the earliest manifestation is widespread pigment clumping at the level of the RPE, which is distinct from the characteristic perivascular bone-spicule pigment clumping seen in retinitis pigmentosa.
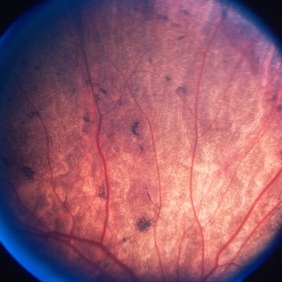
Subsequently, patients develop well-defined regions of atrophy with the visible underlying sclera and large choroidal vessels, most commonly in the post-equatorial region just outside the vascular arcades.
These areas of atrophy advance centripetally and are also found in a peripapillary and parapapillary manner (See image below). An island of foveal tissue may persist until later stages of the disease when central and color vision become affected by foveal atrophy.
Patients have preserved larger choroidal blood vessels and normal-appearing retinal vessels. In addition, choroideremia exhibits no optic atrophy, unlike the waxy pallor of optic discs seen in retinitis pigmentosa. Carrier patients can have mild RPE changes, and in severe cases, patchy RPE degeneration and areas of atrophy.
This phenotypic variability in carriers is due to lyonization in which one copy of the X chromosome is randomly silenced early in embryogenesis. Other associated ocular findings include 31% of patients developing posterior subcapsular cataracts and a small risk of macular edema or choroidal neovascularization.
Choroideremia MANAGEMENT
Choroideremia does not have a specific cure, however, there are treatment options available. Your doctor may prescribe you medications or glasses to manage your symptoms.
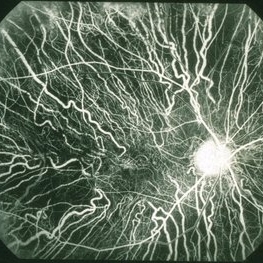
However, with the advancements in science, the newer therapies that have been used to treat choroideremia include:
Gene Therapy:
In this treatment, a trained specialist inserts a gene into the targeted cell or cells to modify the protein that is responsible for the retinal malfunction.
Stem Cell Therapy:
In this treatment, special body cells called stem cells are used to treat retinal dystrophy (degeneration of the cells in the retina). The stem cells have the capacity to develop new tissues and repair damaged or diseased tissues and organs.
Retinal Prosthesis System:
In this treatment, an implantable electronic eye device called a retinal prosthesis is placed in your eye. This retinal prosthesis system has the ability to stimulate the sensation of vision in the eyes of individuals with significant retinal diseases which include choroideremia.
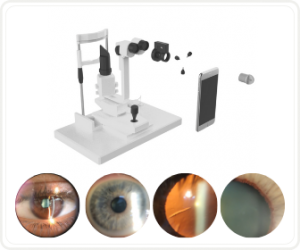
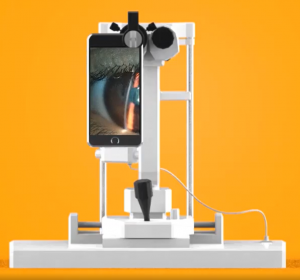
Would you have interest in taking retina images by smartphone?
Fundus photography is superior to fundus analysis as it enables intraocular pathologies to be photo-captured and encrypted information to be shared with colleagues and patients.
Recent technologies allow smartphone-based attachments and integrated lens adaptors to transform the smartphone into a portable fundus camera and Retinal imaging by smartphone.
RETINAL IMAGING BY YOUR SMARTPHONE
REFERENCES
- Welder D, Jeffrey D. Photographer: Toni Venckus. Choroideremia. This image is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.
- Barnard AR, Groppe M, MacLaren RE. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harbor perspectives in medicine. 2014;5(3):a017293.
- Sorsby A, Franceschetti A, Joseph R, Davey JB. Choroideremia; clinical and genetic aspects. The British journal of ophthalmology. 1952;36(10):547-581.
- Cremers FP, Brunsmann F, Berger W, et al. Cloning of the breakpoints of a deletion associated with choroideremia. Human genetics. 1990;86(1):61-64.
- van Bokhoven H, van den Hurk JA, Bogerd L, et al. Cloning and characterization of the human choroideremia gene. Human molecular genetics. 1994;3(7):1041-1046.
RETINAL IMAGING BY YOUR SMARTPHONE
RETINAL IMAGING BY YOUR SMARTPHONE

Thank you for writing this post!