Introduction
Long-term use of pentosan polysulfate sodium (PPS) is strongly linked to a vision-threatening macular disease characterized by a unique constellation of retinal imaging findings.

Pentosan polysulfate sodium (PPS) is the only Food and Drug Administration, USA (FDA) approved oral medication for the treatment of interstitial cystitis (IC).
It has been used extensively to treat IC since its approval in 1996. Several recent studies have described a unique, progressive maculopathy associated in a dose-dependent manner with long-term use of the medication.
It often causes symptoms of prolonged dark adaptation and nyctalopia and may cause blurred vision. While visual acuity may initially remain intact, cystoid macular edema, macular neovascularization, and retinal pigment epithelium (RPE) atrophy may occur resulting in severe vision loss.
Although its pathogenesis has not yet been definitively established, clinical imaging suggests that PPS maculopathy primarily impacts the RPE and the RPE-photoreceptor interface and results in a characteristic pattern of retinal imaging findings.
Pentosan Polysulfate Maculopathy Clinical diagnosis
Clinical diagnosis may be highly suggested in patients with characteristic symptoms, DFE, and a history of extensive PPS use. However, multimodal retinal imaging is typically necessary to definitively diagnose PPS maculopathy.
Characteristic features of PPS maculopathy were defined in one study as the following:
(1) bilateral pathology centered on the fovea.
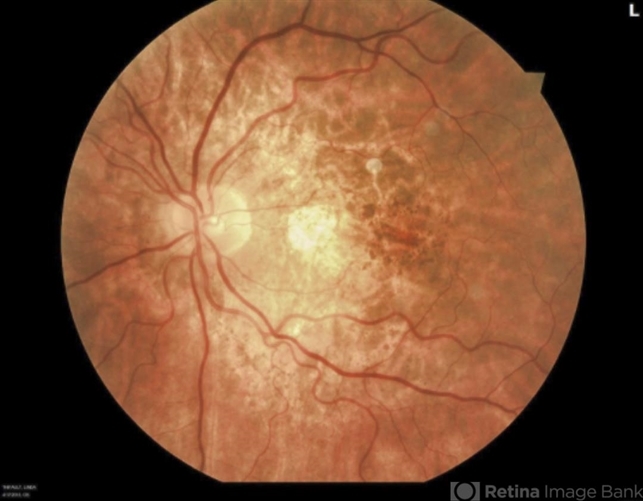
(2) fundus photography revealing paracentral macular hyperpigmented spots, pale yellow deposits, and/or patchy retinal pigment epithelium atrophy.
(3) a dense array of hyper- and hypoautofluorescent spots and reticular fundus autofluorescence imaging abnormalities.
(4) foci of nodular retinal pigment epithelium enlargement on OCT imaging corresponding to hyperreflectance on near-infrared reflectance imaging.
Pentosan Polysulfate Maculopathy Diagnostic procedures
Fundus photography, fundus autofluorescence imaging (FAF), optical coherence tomography (OCT), and near-infrared reflectance imaging (NIR) are useful imaging modalities to establish a diagnosis of PPS maculopathy.
Imaging findings are typically symmetric between both eyes, with rare cases of disease asymmetry. Color fundus photography typically shows more subtle manifestations compared to FAF.
Hyperpigmented macular spots and deep yellowish subretinal deposits may be apparent, particularly in milder cases. Patchy parafoveal RPE atrophy manifests in more advanced cases.
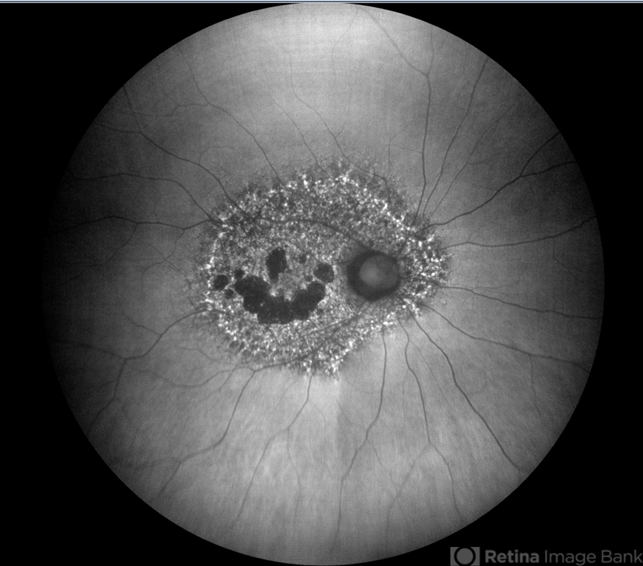
Fundus autofluorescence imaging reveals a striking, densely packed array of hyper- and hypoautofluorescent spots typically centered on and involving the fovea.
Hyperautofluorescent spots colocalize with pigmented spots and yellow subretinal deposits apparent in color fundus photography.
In cases where the disease extends to the peripapillary region, there is typically a hypoautofluorescent peripapillary halo.
RPE atrophy may also be noted in more severe cases, initially as multifocal parafoveal lesions, that ultimately coalesce and encroach on the foveal center. Widefield FAF imaging is helpful in elucidating the extent of involved tissue.
Optical coherence tomography shows hyperreflective nodules at the level of the RPE that colocalize with macular pigment clumps on color fundus photography, hyperautofluorescence on FAF, and hyperreflectance on NIR imaging.
Unlike typical drusen or subretinal drusenoid deposits, these lesions appear to reside at the level of the RPE and project a shadow onto the underlying choroid.
These lesions may not be present on macular OCT in late-stage atrophic disease. Although there may be ill-defined irregularity in the outer retinal bands, there is no clear OCT correlation for yellow macular deposits or the hypoautofluorescent component of FAF lesions.
OCT angiography may demonstrate choriocapillaris flow deficits, which may precede other imaging abnormalities in individuals with high PPS exposure.
Near-infrared reflectance imaging is another important imaging modality with prominent findings that may be present even in the absence of visible lesions in other imaging modalities.
Nodular hyperreflective lesions are visible on NIR that correspond to the pigmented lesions on color fundus photography and hyperreflective RPE excrescences on OCT.
Some functional visual tests may also be useful in detecting visual dysfunction not apparent with BCVA testing alone.
Dark adaptometry, perimetry, electroretinography, and visual function questionnaires such as the National Eye Institute Visual Function Questionnaire-39 and the Low Luminance Questionnaire have shown profound visual function deficits in PPS maculopathy patients that were not fully captured with BCVA testing.
Pentosan Polysulfate Maculopathy Management
There is currently no known treatment for PPS maculopathy. Primary prevention through avoidance or minimizing the cumulative exposure to PPS appears to be key to preventing the condition.
Affected patients should undergo continued monitoring for treatable complications of PPS maculopathy such as CME and MNV, as outlined below.
General treatment:
Prescribers of PPS should consider counseling patients regarding the risk of vision loss associated with PPS and have a discussion weighing the risks and benefits of taking PPS and potential alternative therapies.
If PPS is prescribed, providers should prescribe the minimum dose and duration necessary for disease management.
After a diagnosis of PPS maculopathy, patients are encouraged to work with their prescriber to transition to other IC therapies.
Avoidance of smoking and protection from ultraviolet radiation may be beneficial, as in other degenerative maculopathies.
After diagnosis, providers should consider monitoring patients annually with multimodal retinal imaging so that treatable vision-threatening sequelae may be addressed should they occur.
Pentosan polysulfate maculopathy may progress after drug cessation. In eyes with RPE atrophy, there is progressive growth of the atrophic lesions after drug cessation.
Additionally, some eyes develop new onset atrophy after drug cessation.
Medical therapy
There is no known treatment for PPS maculopathy itself. Affected individuals should consult with their ophthalmologist and PPS prescriber to discuss drug cessation and explore alternative treatments for IC.

Some vision-threatening complications of PPS maculopathy, including CME and MNV, can be treated pharmacologically.
CME can be treated with topical carbonic anhydrase inhibitors, corticosteroids, or nonsteroidal anti-inflammatory agents; oral acetazolamide; or intravitreal anti-VEGF injections.
Macular neovascularization can also be treated with intravitreal anti-VEGF therapy. The maculopathy may worsen after discontinuation of Pentosan Polysulfate which suggests that early screening is important.
Medical follow up
Ophthalmologists at the Emory Eye Center monitor patients taking PPS or diagnosed with PPS maculopathy annually with a comprehensive retinal evaluation including color fundus photography, FAF, OCT, and NIR imaging.
Another group recommends an initial exam within six months of initiating PPS and annual exams as the patient approaches 500 g cumulative exposure.
Another study highlights the importance of increasing awareness among primary care physicians who may be providing prescription renewals.
Complications
Retinal pigment epithelium atrophy appears to be a manifestation of advanced PPS maculopathy. It typically first develops as parafoveal multifocal atrophy that may coalesce over time and involve the foveal center.
Leaking and non-leaking CME have been described in PPS maculopathy and have been successfully treated with both topical and intravitreal therapies described above.
Macular neovascularization has been reported in PPS maculopathy patients and has been successfully treated with intravitreal anti-VEGF injections.
Prognosis
Although more longitudinal data is needed to explore the long-term prognosis of this recently discovered condition, PPS maculopathy is not known to be reversible and may progress after drug cessation.
These findings suggest that early detection is beneficial. Additionally, patients with PPS maculopathy should undergo continued monitoring for treatable complications including CME and CNV.
Would you have interest in taking retinal images with your smartphone?
Fundus photography is superior to fundus analysis as it enables intraocular pathologies to be photo-captured and encrypted information to be shared with colleagues and patients.
Recent technologies allow smartphone-based attachments and integrated lens adaptors to transform the smartphone into a portable fundus camera and Retinal imaging by smartphone.
RETINAL IMAGING BY YOUR SMARTPHONE
References
- Huckfeldt, R. M., & Vavvas, D. G. (2019). Progressive maculopathy after discontinuation of pentosan polysulfate sodium. Ophthalmic Surg. Lasers Imaging Retina, 50(10), 656–659. https://doi.org/10.3928/23258160-20191009-10
- Lyons, R., Hanif, A., & Jain, N. (2020). Pentosan Polysulfate Maculopathy: Comprehensive Functional Analysis and Structure-Function Correlation. Invest Ophthalmol Vis Sci, 61(7), 1068–1068.
- Christiansen, J. S., Barnes, A. C., Berry, D. E., & Jain, N. (2022). Pentosan polysulfate maculopathy versus age-related macular degeneration: comparative assessment with multimodal imaging. Canadian Journal of Ophthalmology, 57(1), 16–22. https://doi.org/10.1016/j.jcjo.2021.02.007
- Jung, E. H., Lindeke-Myers, A., & Jain, N. (2023). Two-year outcomes after variable duration of drug cessation in patients with maculopathy associated with pentosan polysulfate use. JAMA Ophthalmol., 141(3), 260–266. https://doi.org/10.1001/jamaophthalmol.2022.6093
- Barnes, A. C., Hanif, A. M., & Jain, N. (2020). Pentosan polysulfate maculopathy versus inherited macular dystrophies: Comparative assessment with multimodal imaging. Ophthalmol. Retina, 4(12), 1196–1201. https://doi.org/10.1016/j.oret.2020.05.008
- Tripathy, K., Sarma, B., & Mazumdar, S. (2020). Outer retinal tubulation and inner retinal pseudocysts in a patient with maternally inherited diabetes and deafness evaluated with optical coherence tomography angiogram. Indian J. Ophthalmol., 68(1), 250–253. https://doi.org/10.4103/ijo.IJO_577_19.
RETINAL IMAGING BY YOUR SMARTPHONE