CASE REPORT
A 47-year-old male with no significant systemic or ocular medical history and no family history of ocular diseases presented with a chief complaint of visual distortion in his right eye, described as wavy lines and a small central blind spot persisting for 6 months.
Clinical evaluation revealed visual acuity of 20/40 in the right eye and 20/20 in the left eye, with normal intraocular pressure.
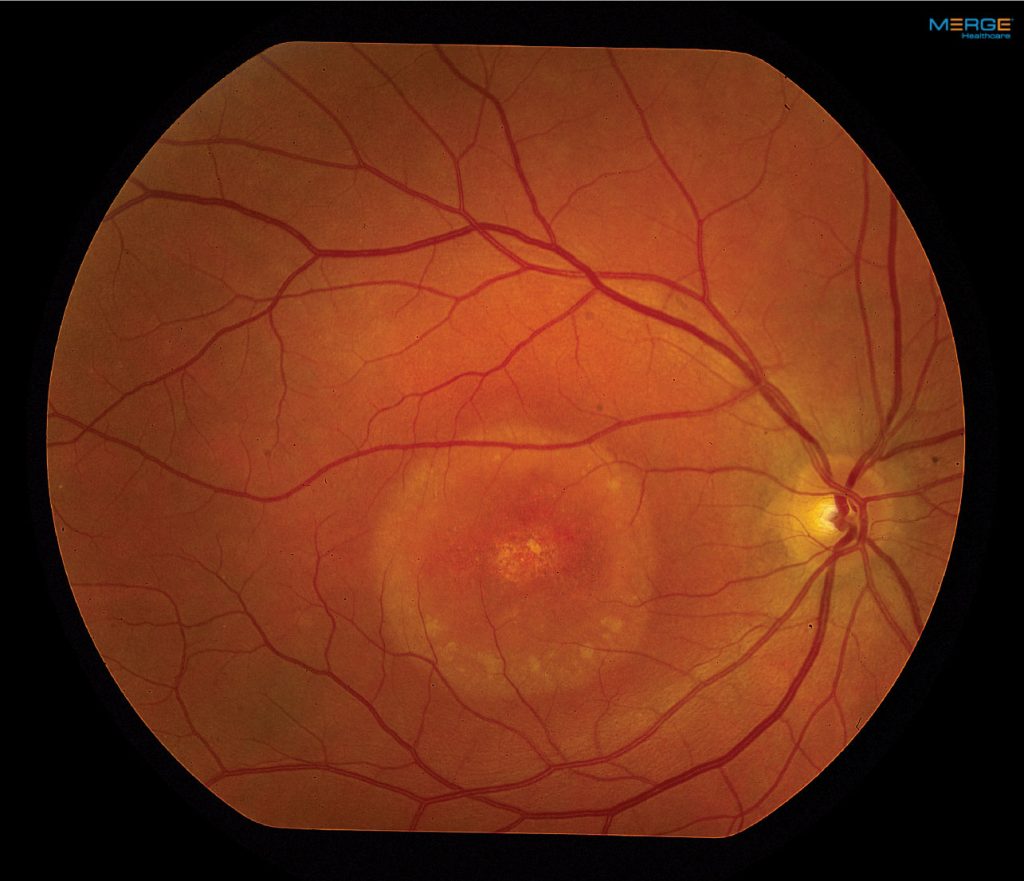
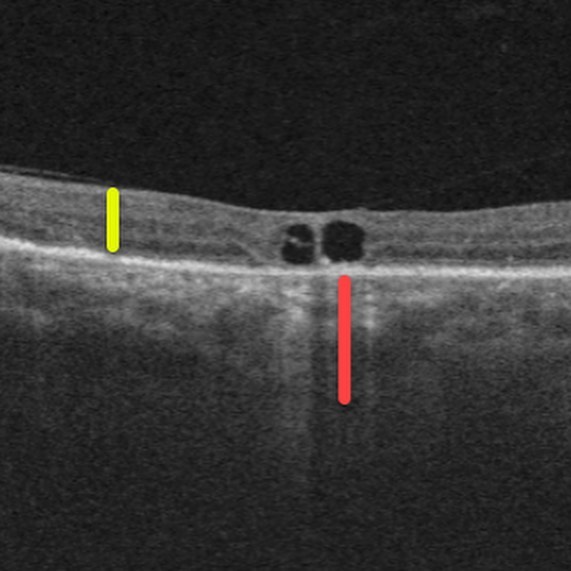
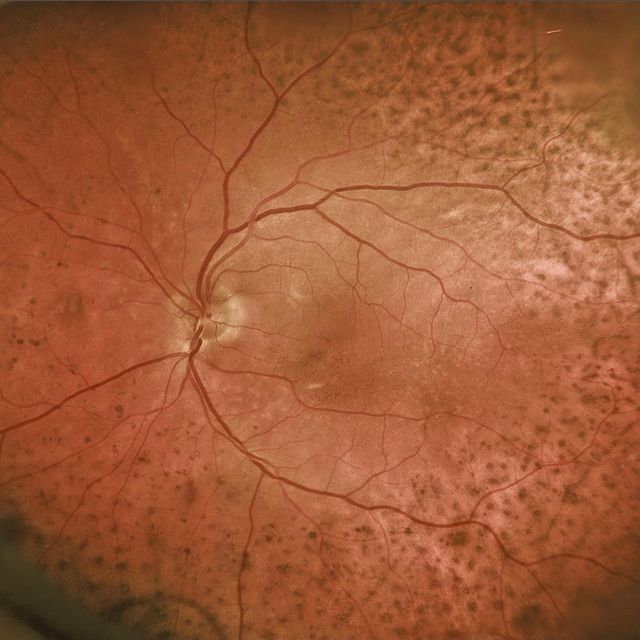
Fundus examination of the right eye showed peripapillary intrachoroidal cavitation (PICC) and subretinal fluid near the optic disc, while the left eye exhibited no significant abnormalities.
Optical coherence tomography (OCT) demonstrated choroidal thickness exceeding 400 microns in the right eye, indicative of pachychoroid, with subfoveal choroidal thickness measuring 460 microns, while the left eye showed normal choroidal thickness.
Fluorescein angiography (FA) in the right eye revealed no signs of choroidal neovascularization, with normal FA findings in the left eye. Consequently, the patient was diagnosed with Peripapillary Pachychoroid Syndrome (PPS) in his right eye.
Peripapillary Pachychoroid Syndrome DISEASE entity
Peripapillary Pachychoroid Syndrome (PPS) is a distinct PDS variant, in which peripapillary choroidal thickening is associated with nasal macular intraretinal and/or subretinal fluid and occasional disk edema.
Recognition of Peripapillary Pachychoroid Syndrome (PPS) is important to distinguish it from disorders with overlapping features such as posterior uveitis and neuro-ophthalmologic conditions.
Peripapillary Pachychoroid Syndrome (PPS) has been recently identified as a distinct Pachychoroid Disease Spectrum (PDS) variant, in which pachychoroid features surround the optic nerve and are associated with the accumulation of intraretinal and/or subretinal fluid in the nasal macular region rather than the fovea, extending from the temporal margin of the optic disk, as well as with occasional optic nerve head edema in some eyes.
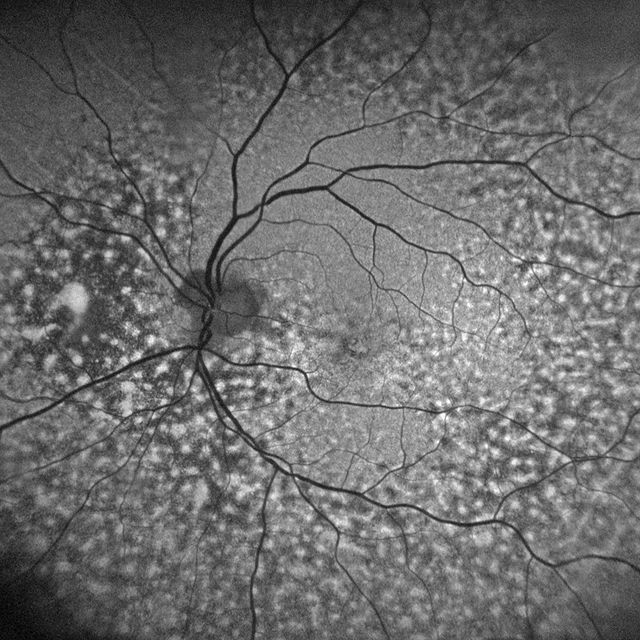
Associated clinical findings include peripapillary choroidal thickening associated with Haller vessel dilation (pachyvessels) and overlying inner choroidal thinning., serous pigment epithelial detachment, and choroidal hyperpermeability, all of which are indicative of a pachychoroid-driven entity, rather than a variant of uveal effusion syndrome (UES) as was suggested in a single-case report.
Most of the eyes have hyperopic refraction and short axial lengths Other common features present in patients with PPS are choroidal folds (77%), short axial lengths (39% less than 23 mm), and hyperopic refractive error (86%), older age, and a small cup-to-disc ratio.
This entity is seen more commonly in males and is usually bilateral. Govetto et al described a sporadic association of Peripapillary Pachychoroid Syndrome (PPS) with pulmonary arterial hypertension and published a case study, in which choroidal ischemia is thought to have been caused by hemodynamic disturbances due to increased central venous pressure.
The pachychoroid disease spectrum (PDS) refers to a group of retinochoroidal disorders, which share distinctive choroidal findings identified with multimodal retinal imaging, which include focal or diffuse choroidal thickening associated with reduced fundus tessellation, dilated Haller layer vessels (termed “pachyvessels”) with thinning of the overlying inner choroid, and choroidal hyperpermeability demonstrated with indocyanine green angiography (ICGA).
The PDS includes pachychoroid pigment epitheliopathy, focal choroidal excavation, central serous chorioretinopathy (CSC), pachychoroid neovasculopathy, polypoidal choroidal vasculopathy, and recently described peripapillary pachychoroid syndrome.
Peripapillary Pachychoroid Syndrome MANAGEMENT
General treatment
CSC- Spontaneous resolution of the disease occurs in 90% of the cases. However, recurrence occurs in around 50% of the cases after resolution of subretinal fluid.
In patients with exogenous steroid intake, the steroids should be stopped or tapered as needed. Treatment is required in cases with a duration greater than 3 months (chronic), highly symptomatic patients, or patients with poor vision in the fellow eye.
The treatment type is governed by the phase of the disease and the site of leakage on FA.
Laser photocoagulation is considered for the treatment of extrafoveal leakage points in acute cases. Focal thermal coagulation seals the RPE defects and prevents further accumulation of subretinal fluid.
However, focal laser photocoagulation causes permanent damage to the RPE and overlying photoreceptors and also carries the risk of iatrogenic CNV formation especially if the laser spot size is less than 100 microns.
To overcome these shortcomings of conventional laser systems, a sub-threshold micropulse (STM) laser was introduced. STM laser does not cause thermal damage to the RPE.
Instead, it stimulates the proliferation of RPE cells. Since it is not destructive and does not produce visible burns/scars, STM laser may be used for even juxtafoveal leaks.
Yellow wavelength (577nm) is often used for STM laser in CSC. The limitation of STM laser is reduced efficacy in chronic cases with diffuse retinal pigment epitheliopathy.
This is because STM acts at the level of RPE and does not alter the dysfunctional hyperpermeable choroidal vasculature.
Verteporfin photodynamic therapy (PDT) is the preferred treatment for chronic cases. PDT targets the choroidal hyperpermeability, reduces the dilated choroidal vasculature, reduces the CT, and leads to the resolution of subretinal fluid.
PDT significantly decreases the thickness of Haller’s layer without alteration of the choriocapillaris and Sattler’s layer, thereby restoring the normal choroidal morphology.
Conventional full dose and full laser fluence PDT may have certain adverse effects such as transient visual disturbances, unexplained visual loss, diffuse RPE atrophy, CNV formation, and choroidal ischemia.
To improve the safety, half dose and/ or half fluence PDT have been advocated, but the efficacy remains questionable.
However, half-dose PDT has shown promise in attaining better final visual acuity and lesser recurrence than conventional PDT in a few studies. Since aberrant steroid metabolism is thought to be the underlying cause of CSCR, numerous systemic drugs have been tried with equivocal results.
Mineralocorticoid receptors in the choroidal vasculature increase the congestion upon stimulation, therefore antagonists such as spironolactone and eplerenone may have a role in treatment. Rifampicin, a mineralocorticoid antagonist, potentiates the catabolism of endogenous steroids and has been reported to accelerate the resolution of subretinal fluid.
However, the majority of the studies reporting the benefits of systemic therapy in chronic CSC lack a control group or have a small sample size.
There is no level 1 evidence to support the use of anti-VEGF agents in the treatment of persistent fluid in chronic CSC cases in the absence of underlying CNV.
Would you have interest in taking retinal images with your smartphone?
Fundus photography is superior to fundus analysis as it enables intraocular pathologies to be photo-captured and encrypted information to be shared with colleagues and patients.
Recent technologies allow smartphone-based attachments and integrated lens adaptors to transform the smartphone into a portable fundus camera and Retinal imaging by smartphone.
RETINAL IMAGING BY YOUR SMARTPHONE
REFERENCES
- Phasukkijwatana, N., Freund, K. B., Dolz-Marco, R., Al-Sheikh, M., Keane, P. A., Egan, C. A., … Sarraf, D. (2018). PERIPAPILLARY PACHYCHOROID SYNDROME. Retina, 38(9), 1652–1667. doi:10.1097/iae.0000000000001907
- Moraru, A.D., Costin, D., Moraru, R.L., Costuleanu, M., & Brănișteanu, D.C. (2020). Current diagnosis and management strategies in pachychoroid spectrum of diseases (Review). Experimental and Therapeutic Medicine, 20, 3528-3535. https://doi.org/10.3892/etm.2020.9094
- Govetto A, Sarraf D, Scialdone A. “HIDE AND SEEK” NEUROSENSORY RETINAL DETACHMENTS IN PERIPAPILLARY PACHYCHOROID SYNDROME ASSOCIATED WITH PULMONARY ARTERIAL HYPERTENSION. Retin Cases Brief Rep. 2019 Nov 21. doi: 10.1097/ICB.0000000000000942. Epub ahead of print. PMID: 31764883.
- Phasukkijwatana N, Freund KB, Dolz-Marco R, Al-Sheikh M, Keane PA, Egan CA, et al. Peripapillary Pachychoroid Syndrome. Retina (Philadelphia, Pa), 2018; 38(9):1652-67.
- Mazzeo, T.J.M.M., Leber, H.M., da Silva, A.G. et al. Pachychoroid disease spectrum: review article. Graefes Arch Clin Exp Ophthalmol 260, 723–735 (2022).
- Castro-Navarro, V., Behar-Cohen, F., Chang, W. et al. Pachychoroid: current concepts on clinical features and pathogenesis. Graefes Arch Clin Exp Ophthalmol 259, 1385–1400 (2021). https://doi.org/10.1007/s00417-020-04940-0.
RETINAL IMAGING BY YOUR SMARTPHONE
RETINAL IMAGING BY YOUR SMARTPHONE